Anti-aging skincare seen through collagen fibres organisation
Novitom has developed an original test based on the…
Home » Articles and News » Quantifying dermal collagen organisation with AI-assisted AFM
Understanding how collagen organisation changes with age remains a major challenge in skin research. While macroscopic alterations can be identified at the fibre bundle scale using conventional histology, many of the most significant age-related modifications occur at a much finer level—within the collagen microfibrils themselves. Capturing and quantifying these subtle structural changes requires both high-resolution imaging and robust analytical tools.
At Novitom, we have developed an AI-assisted Atomic Force Microscopy (AFM) approach that enables objective, quantitative assessment of collagen organisation in the dermis at the interfibrillar scale.
This methodology, presented at IFSCC 2025 in collaboration with Clarins, opens new perspectives for evaluating skin ageing models and the efficacy of anti-ageing treatments.
Collagen microfibrils are arranged in highly ordered, parallel structures characterised by their distinctive 65 nm banding pattern. With ageing, processes such as glycation and carbamylation progressively disrupt this organisation, leading to reduced cohesion, increased disorder and, ultimately, altered mechanical and biological properties of the dermis.
Traditional histological techniques are effective at detecting changes at the fibre bundle level, but they lack the resolution required to characterise microfibrillar architecture. Techniques such as transmission electron microscopy (TEM) or AFM provide access to this scale; however, image interpretation has often relied on subjective visual assessment or limited orientation metrics.
To address these limitations, Novitom has developed an AI-based image analysis framework capable of transforming AFM images into meaningful, quantitative descriptors of collagen quality.
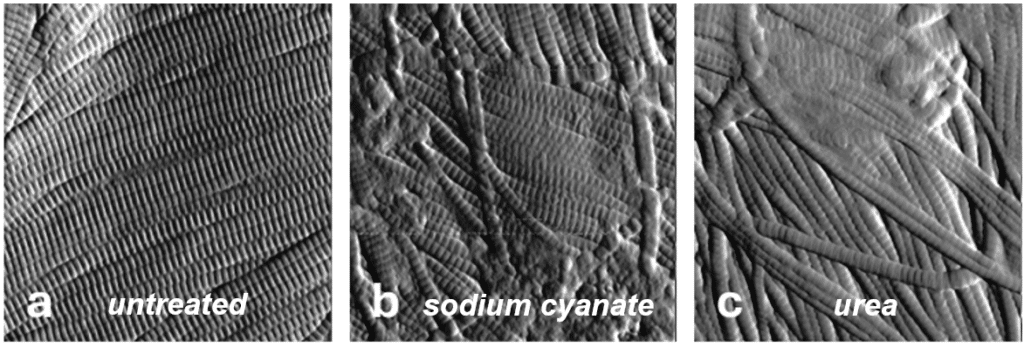
To validate this approach, human skin explants were subjected to controlled ageing through carbamylation, a well-established chemical model of dermal ageing. Three conditions were investigated:
Untreated explants
Carbamylation induced by sodium cyanate
Carbamylation induced by urea
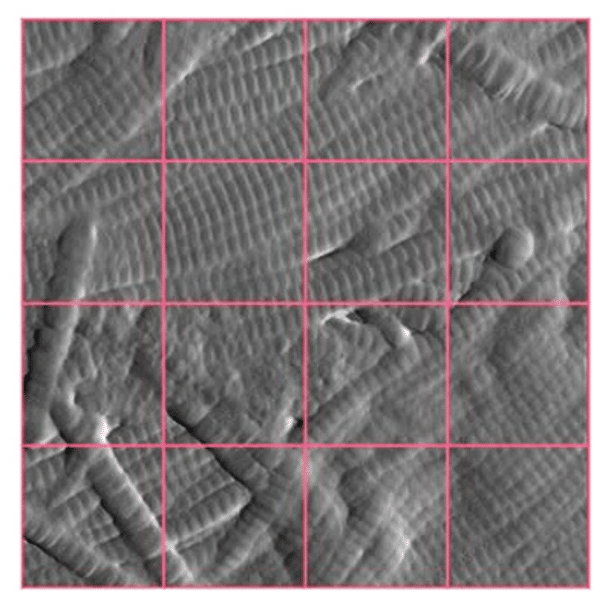
Following repeated treatments over 13 days under survival conditions, the explants were cryofixed and sectioned. AFM imaging was then performed in the dermis, 500 µm below the epidermis, using high-resolution scans (3 × 3 µm²) to capture collagen microfibril organisation.
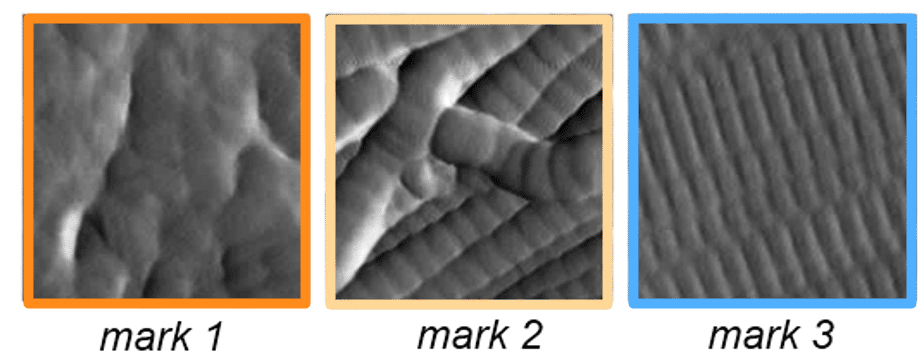
Each AFM image was subdivided into 16 smaller regions (0.75 × 0.75 µm²). A neural network developed using Novitom software was trained to recognise and score three distinct types of collagen organisation:
Mark 1 – Disorganised microfibrils
Mark 2 – Criss-crossed microfibrils
Mark 3 – Rectilinear, parallel microfibrils (well-organised collagen)
Despite the limited size of the training dataset, the model achieved a 95% prediction success rate, demonstrating its robustness and suitability for this application. Once trained, the AI automatically analysed all sub-images, generating both distribution profiles and global scores for each explant condition.
The results highlight the sensitivity of the AFM/AI approach:
Untreated explants showed a very high proportion of well-organised collagen, with 94% of sub-images classified as Mark 3.
Carbamylated explants displayed a marked shift towards disorganised and intermediate structures, with Marks 1 and 2 more evenly distributed.
The AI-derived global score clearly quantified these differences:
Untreated: 2.91
Carbamylated (sodium cyanate): 2.11
Carbamylated (urea): 2.14
These findings objectively demonstrate carbamylation-induced alterations in collagen microfibril organisation—changes that are often difficult to quantify using conventional analytical methods
Novitom has developed an original test based on the…
X-ray diffraction reveals the structural organisation of the intercorneocyte…